Liposome manufacturing under continuous flow conditions: towards a fully integrated set-up with in-line control of critical quality attributes
Maryam Sheybanifard, Luis P. B. Guerzoni, Abdolrahman Omidinia-Anarkoli, Laura De Laporte, Johannes Buyel, Rut Besseling, Michiel Damen, Ad Gerich,h Twan Lammers a and Josbert M. Metselaar*
Continuous flow manufacturing (CFM) has shown remarkable advantages in the industrial-scale production of drug-loaded nanomedicines, including mRNA-based COVID-19 vaccines. Thus far, CFM research in nanomedicine has mainly focused on the initial particle formation step, while post-formation production steps are rarely integrated. The opportunity to implement in-line quality control of critical quality attributes merits closer investigation.
Here, we designed and tested a CFM setup for the manufacturing of liposomal nanomedicines that can potentially encompass all manufacturing steps in an end-to-end system. Our main aim was to elucidate the key composition and process parameters that affect the physicochemical characteristics of the liposomes.
Such in-line measurements allow for real-time monitoring and in-process adjustment of key composition and process parameters.
Read the full article:
Free download article Sheybanifard et al.
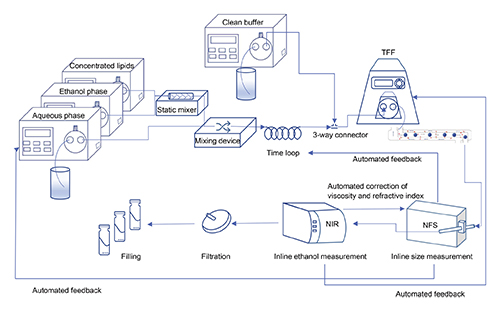


Up to millions is lost per year due to the absence of appropriate
particle size monitoring solutions…