Continuous processing and enhanced control of lipid-based nanoparticles: LNP’s and liposomes
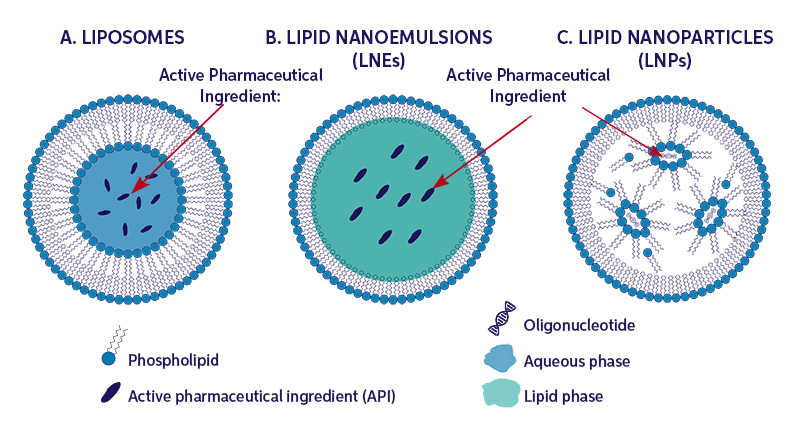
Nanoparticles have once again reached the spotlight, this time as a critical tool to combat the spread of covid-19. In this particular case, messenger ribonucleic acid (mRNA) encapsulated in lipid nanoparticles (LNPs) has been successfully manufactured, distributed and administered to populations throughout the world.
The high efficacy and strong protection of the COVID-19 mRNA-LNP vaccines have made them ideal candidates for future vaccines and therapeutics. However, information released by the EMA COVID-19 data leak emphasizes the need to better understand quality issues observed throughout the LNP manufacturing process. Quality issues may be traced to RNA instability and LNP particle size. In this article, process analytical technology and continuous processing of nanoparticles will be introduced as a means to provide the necessary quality required for these injectable, complex formulations.
Read the full whitepaper:


Up to millions is lost per year due to the absence of appropriate
particle size monitoring solutions…